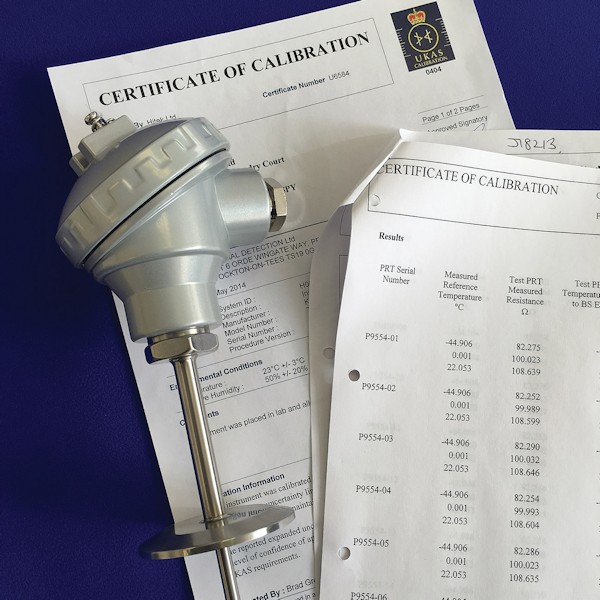
Pharmaceutical Process Pocket with Insert (MDP)
For temperature measurement on pharmaceutical processes, manufacturing and service plant. The assembly has a rugged construction and allows for the replacement of the sensor insert whilst the main assembly remains connected to the process, eliminating process down time.
RTD format PT100: Class B, A, AA as IEC 60751 also 1/10 DIN
RTD format PT1000: Class B, A
Thermocouple Type T (to IEC 60584-1).
The MDP is supplied in two parts: the thermowell/pocket with FDA approved terminal head for transmitter or terminal block, and a removable spring loaded insert containing the temperature sensor. The pocket can be specified in a wide range of sizes, with optional process connection and instrument connection options.
Product Features
- All metallic parts in Grade 316L (1.4404/1.4435) stainless steel only.
- Supplied as two pieces. Thermowell and Spring loaded insert
- Insert available with
- PT100 or PT1000 RTD (PT100 as Class A, AA as per IEC 60751 also 1/10 DIN, PT1000 Class A)
- In thermocouple type T as per IEC 60584-1.
- Plain stem pocket/thermowell as standard (MDP) or with fixed screwed or compression fitting (MDPS)
- Units are available with a temperature transmitter to provide a signal output of 4 to 20mA for transmission to an indicator, recorder, data-logger or other instrumentation.
- Standard connection head is in white polypropylene and FDA approved with a screw cap and retention chain and an M20 cable entry. Environmental rating of IP67.
- Can also be supplied with a grade 316 stainless steel connection head.