Pharmaceutical Steam Quality Test Kits (SQTK)
High quality steam is an essential requirement for maintaining optimum results in steam sterilisation cycles.
The testing of steam supply to sterilisers used in the pharmaceutical, biotech and healthcare industries, has been an EU requirement for many years, as specified in EN 285. The tests specified in EN 285 have been adopted by many organisations worldwide.
The tests are designed for the following measurements (see below for an overview of each test):
- Non condensable gas.
- Steam superheat.
- Steam dryness value.
- Steam sample for laboratory analysis.
Our range of steam quality test kits are designed with ease of use in mind. The kit components have been chosen to ensure all the necessary equipment is contained in one case that can be easily transported onto site.
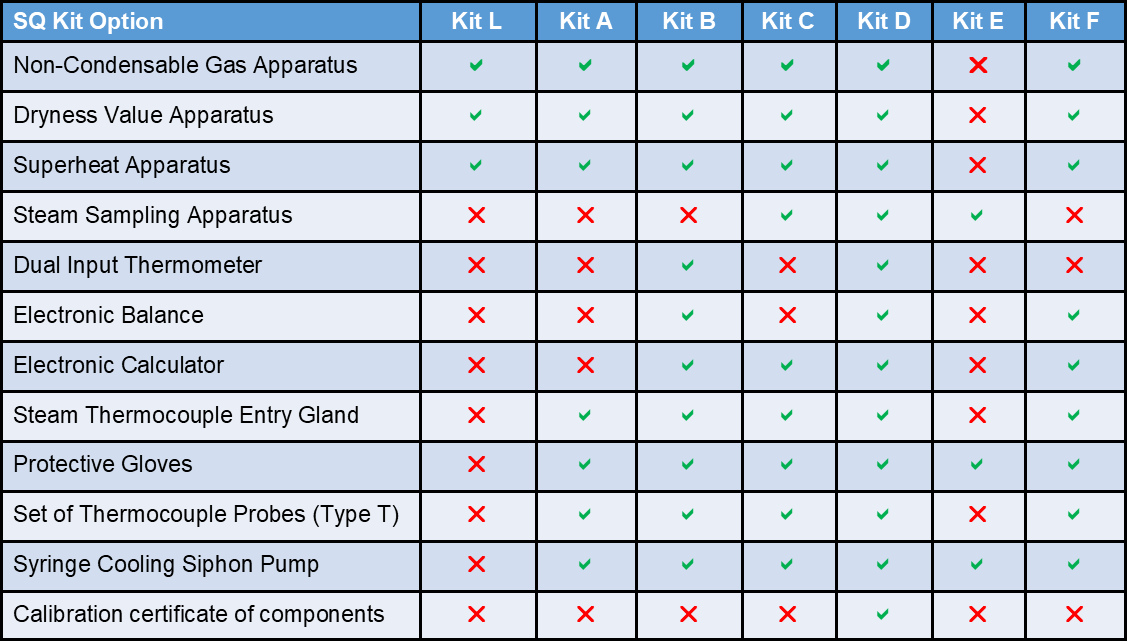
There are seven kit options, from the Lite kit (minimum equipment), to the full Kit D (all equipment), these are shown in the table below.
Non Condensable Gas Test

The non-condensable gas test is designed to demonstrate that the attainment of sterilisation conditions in all parts of a steriliser load (particularly for porous load items) is not impaired by the presence of non-condensable gases.
The measurement of non-condensable gases is made by cooling a steam sample, using water through an efficient condenser. Water can be supplied either directly from a pressurised supply or simply by siphoning from a tank at a flow rate of 200ml/minute, provided that a minimum head of 1.0 metre is maintained and at a temperature not exceeding 25°C.
When the sampled steam is condensed, any non-condensable gases that may be present are liberated and separated from the produced condensate into two sight glass columns. The gas and steam condensate volumes are measured by the ‘zero adjustable’ scale mounted behind the two sight glasses.The temperature of the condensate is maintained above 80°C by controlling the steam flow through the inlet needle valve whilst measuring the temperature of the condensate via a thermocouple fitted to the outlet of the condenser.To carry out repeated tests, a return to the ’zero scales’ position is made by opening the condensate drain and gas bleed valves. The valves are then closed when a new sample is required to be taken.
Volumes up to 14ml of gas and 140ml of condensate are possible for each sample.
The results of the non-condensable gas test are considered to be acceptable for sterilisation purposes on clinical sterilisers if the percentage of gas to condensate is less than 3.5%.In addition to calculations on the memory stick, an electronic calculator and table are provided to conveniently calculate the percentage of non-condensable
Steam Superheat

The purpose of the test is to demonstrate that the amount of moisture in the steam supply is sufficient to prevent the steam from being superheated as it enters the expanded space of a sterilizer chamber.
For this test the temperature of steam passing through an orifice in a pitot tube to atmosphere is measured.
The temperature is measured by using a thermocouple which is located at the centre of the expansion tube placed over the pitot tube. The temperature of the steam is considered to be acceptable if it is less than 25°C above that of the local temperature of boiling water, which is altitude dependant.
Steam Dryness Value

The purpose of the steam dryness value test is to ensure that an acceptable amount of moisture is present in the steam supply.
Too little moisture:
- Possibility of superheating
- Prevents optimum sterilisation conditions in the load as moisture is a critical factor for breaking down cell structures of sporing organisms.
Too much moisture:
- Steriliser load may not be acceptably dry at the end of the cycle. Damp wrapping material can cause a break in the microbiological barrier providiing a path for re-contamination.
The test is performed by a heat balance using a stainless steel vacuum flask. The flask is primed with a known mass of water at a known temperature. Steam is then condensed in the flask thus raising the temperature of the water. The final mass and temperature of the water are measured and placed into a formula. If the water temperature is lower or the final mass greater, this would indicate that the steam had a higher moisture content, i.e. having a lower value.
Whilst this method is not regarded as a truly accurate measurement of moisture in the steam, it can be used to demonstrate acceptable or otherwise dryness for sterilisation purposes. A dryness value of 1.0 is equivalent to ’dry saturated steam’. For sterilisation purposes a factor of 0.9 is acceptable for non-metal steriliser loads and for loads containing metal, a factor of 0.95.
As the flask has to be connected within 500mm of the sampling point, the test kit incorporates a flask holder so that the flask can be mounted in a convenient position. This holder also serves as a measuring container to prime the flask.
For the engineers convenience, the calculation can be performed manually or by the use of an Excel spread sheet. An electronic scientific calculator and flash drive containing all forms and calculations formulae are supplied.
Steam Sample Collection
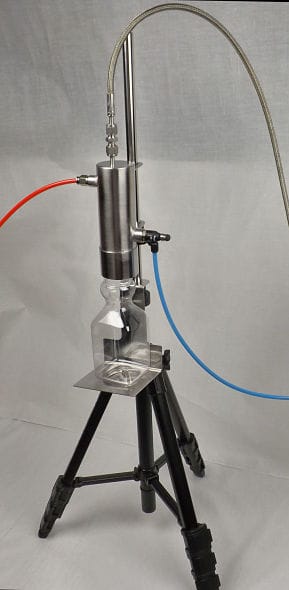
As the steam supply is in direct contact with items that may, in healthcare applications, be in direct contact with patients, any contaminants present in the steam supply could cause adverse effects.
Surgical instruments and items used for intravenous injection could effectively be introducing these contaminants into the patients body cavities. Thus resulting in the body’s normal immune system being bypassed.
For the clean steam test, a sample of condensed steam is collected with depyrogenated equipment into a sterile pyrogens free container. The sample is then analysed by a laboratory to determine the level of chemical impurities and bacterial endotoxins.
The 316 stainless steel, clean steam condenser is very efficient and compact and the condensate sample is collected cleanly due to the design shape of the condenser outlet. The condenser, pitot tube, isolating valve and steam supply tubing is required to be depyrogenated before use and this can be carried out in a suitably dry heat steriliser/oven at 180°C for three hours prior to their use.
Product Features
- Seven kits to choose from
- Portable and housed within a compact case or flight case
- Offers various options to suit all requirements:
- Non condensable gas value.
- Steam superheat value.
- Steam dryness value.
- Clean steam analysis.
- All procedures meet the current European Standard of EN285 and the UK Health Service Standard HTM2010 and HTM2031.
- Hygienic test elbows in 316L stainless steel available in ¾", 1", 1½", 2" and 3" pipe sizes.
- Other accessories available on application.